Process deviations pose critical risks across all science-led environments. While pharmaceutical production often grabs headlines when things go wrong, the reality is that laboratories conducting analytical testing, research facilities pushing the boundaries of discovery, and specialized environments handling sensitive materials all face similar challenges. From misplaced or mislabeled samples to overlooked procedural steps, even the smallest deviation can cascade into wasted time, lost resources, or setbacks that take years to recover from. This article examines how real-time monitoring can prevent these costly consequences across the full spectrum of science-based operations, from regulated manufacturing floors to university research labs.
Why no deviation is ever minor in pharma manufacturing
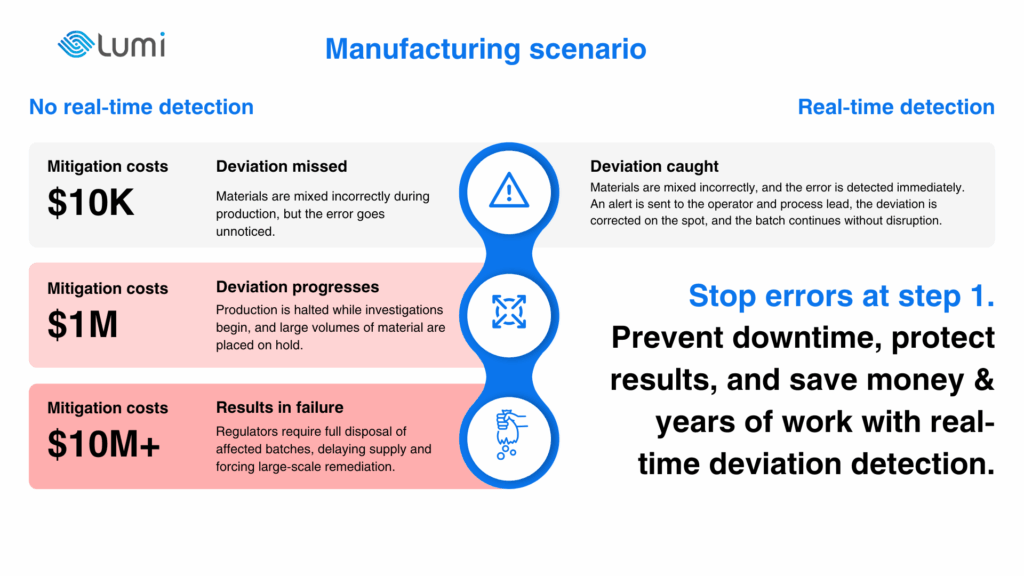
In regulated manufacturing, deviations that appear minor at first glance often trigger the most severe consequences. A shift in operator movement within a cleanroom, a fume hood sash left at the wrong height, or a small lapse in gowning procedure can all lead to contamination, forcing entire batches to be discarded and production halted while lines are deep cleaned.
Process parameters are another critical point. A deviation in something like temperature, agitation speed, or humidity, even for a short period, can alter product stability, physical form, impurity profile or potency. Due to the fact that humans aren’t perfect tools for 360 awareness, these issues are often discovered late in the workflow, which can result in repeated runs and weeks of delay. Cleaning itself is also a source of risk: in some sterile and biologics facilities, it may consume up to 30 per cent of production time, and any failed step or poor aseptic technique means idle manufacturing capacity until revalidation is completed.
When deviations of this kind go unnoticed, the outcome can vary from minor rework to batch failure, or even result in the release of unacceptable material to patients with all the legal and reputational damage that can entail. Even a modest reduction in failure rates can preserve millions in revenue, underlining the high cost of missing what might at first look like minor errors. Together with the loss of money comes the loss of time – and it is not only the time needed to resolve the issue. In some cases, deviations can push regulatory timelines off track, with the FDA placing product approvals on hold for months. For manufacturers, that delay can be as damaging as a full production stop, stalling both market entry and revenue.
Sample and material tracking: when an error turns into horror
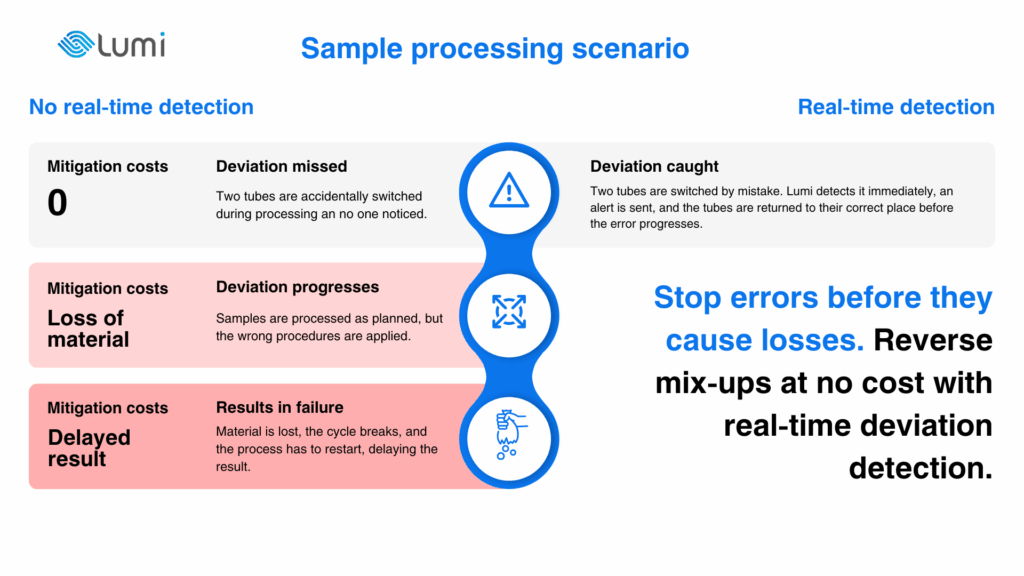
Analytical and testing facilities that handle critical samples face a unique risk profile. A single misplaced or mislabelled vial can produce a false result, or no result at all if the material is lost. In these environments, there are almost no harmless mistakes. A blood sample mixed up or processed incorrectly can lead to misdiagnosis, while in a forensic lab, the same error might result in a miscarriage of justice. In reproductive medicine, losing or contaminating a sample can compromise an IVF cycle. In drug development, the loss of patient-specific material can derail an entire personalised treatment pathway.
The cost here is not only financial. Every misplaced or mislabelled sample represents wasted time, delayed answers, and in many cases, irreversible consequences for patients or clients relying on the accuracy of the result.
Research: When a single slip derails discovery
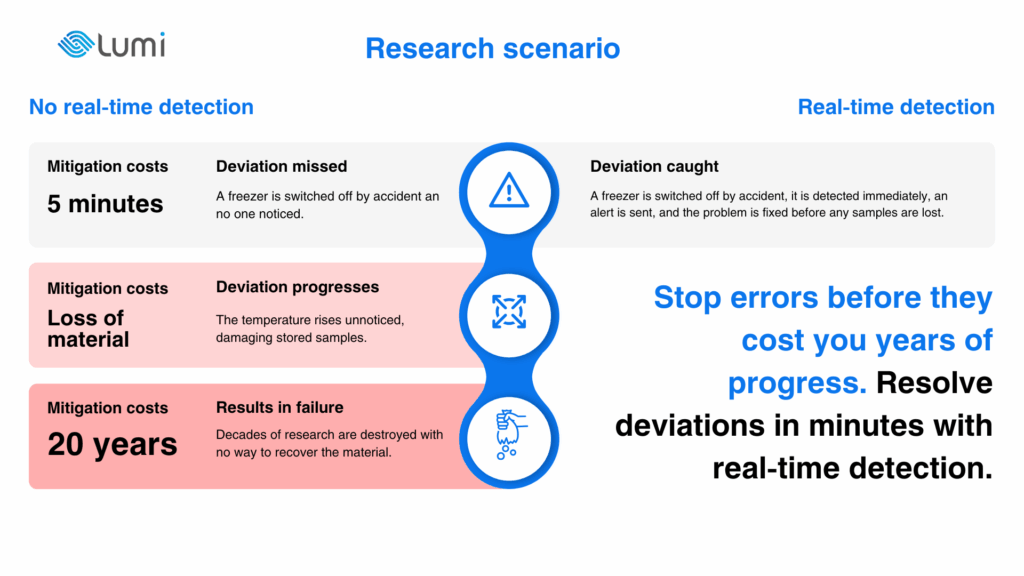
Last but not least on the deviation horror list is research itself. Here, the stakes are often hidden until it is too late. A fridge left open by accident once destroyed five years of work for a PhD researcher. In another case, a procedural error misled scientists with false results and delayed a discovery by two decades.
These are not isolated stories. At Rensselaer Polytechnic Institute, a freezer accidentally switched off by a cleaner wiped out over 20 years of cell culture research. At Harvard, a freezer malfunction destroyed 147 donated human brains, including 54 from individuals with autism, setting autism research back by almost a decade. In Queensland, a biosecurity lapse led to more than 300 virus samples going missing, including highly dangerous pathogens, sparking a full-scale investigation.
Early-stage research is often under-resourced and depends heavily on one person or a very small team. That concentration of responsibility means even the smallest deviation – an overlooked step, a mistimed process, or a forgotten control – can erase years of effort and push entire fields of knowledge backwards.
How real-time deviation detection changes the game
When things go wrong, the blame often falls on people: someone did not notice, did not act in time, or did not escalate properly. But the deeper issue is the lack of tools to support them. Perfect recall is rare, even in the best-funded facilities staff are stretched thin, and mistakes are inevitable.
Over the past five years, we have implemented Lumi across different science-led environments, and real-time monitoring has consistently been one of the strongest drivers of adoption. By using live video streams as the source of data, Lumi can detect a deviation the moment it happens and notify both the operator and the person responsible for process control. Whether it is a procedural slip or an operational drift, the deviation is caught before it progresses further and escalates into a full-scale error.
Unexpected or out-of-spec results usually trigger lengthy investigations, often based on incomplete notes. Real-time deviation detection provides a richer record of what actually happened, making it faster and easier to identify the true cause, and preventing small errors from escalating.
The benefit here is clear: it stops at step one. Instead of a contaminated production line, it’s a quick clean-up. Instead of a false test result, it’s a sample put right back where it’s supposed to be. And instead of five years down the drain, it’s a simple notification to close the fridge.
Step 0 starts here: book a chat with us and discover how Lumi can support your work.