Introduction
Regulatory compliance is a key concern for industries ranging from pharmaceuticals to clinical research. Ensuring adherence to industry standards such as Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) demands robust data integrity and traceability. Lumi offers a verifiable audit trail that seamlessly integrates compliance into laboratory or manufacturing operations. Every action – whether adding a reagent, recording an instrument reading, or executing a procedural step – is automatically logged, timestamped, and securely stored.
This step-by-step implementation scenario illustrates how Lumi enhances data integrity and traceability, ensuring a higher level of compliance while reducing manual workload and human errors.
Step 1: System integration & setup
Lumi is designed to integrate seamlessly with laboratory and manufacturing environments. Implementation involves:
- Setting up Lumi’s monitoring system in any areas that require monitoring
- Connecting Lumi to existing software or hardware solutions
- Configuring access permissions for key stakeholders
The setup is very simple – a company can opt for either using LabEye modules that can be placed almost anywhere (see the example below) or use existing recording devices (such as security cameras, webcams, etc.).
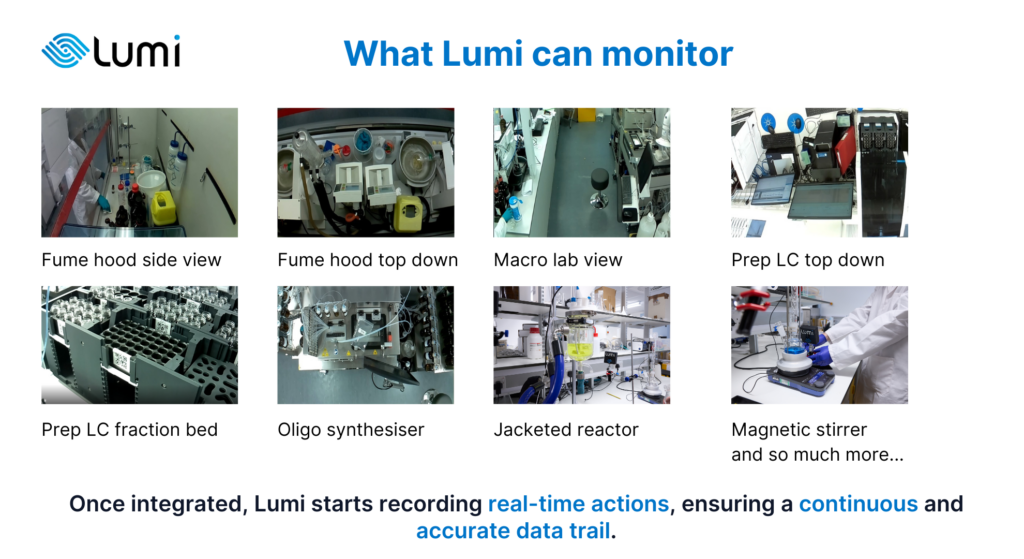
Once implemented, Lumi starts recording real-time actions, ensuring a continuous and accurate data trail.
Step 2: Real-time data capture & verification
Lumi captures every visible action within the monitored environment, providing:
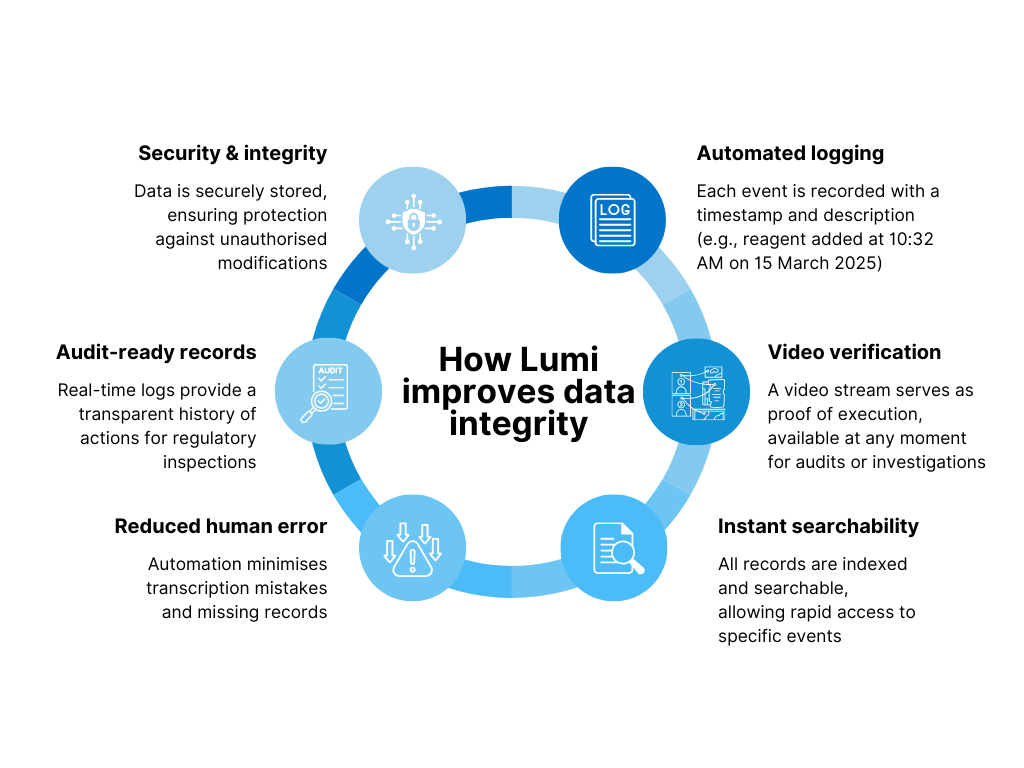
- Automated logging: Each event is recorded with a timestamp and description (e.g., reagent added at 10:32 AM on 15 March 2025)
- Video verification: A video stream serves as proof of execution, available at any moment for audits or investigations
- Instant searchability: All records are indexed and searchable, allowing rapid access to specific events
This ensures complete traceability and eliminates the risks of missing or altered data.
Step 3: Enhancing compliance readiness
Lumi simplifies compliance by ensuring all data is structured, verifiable, and aligned with regulatory expectations. Key benefits include:
- Reduced human error: Automation minimises transcription mistakes and missing records
- Audit-ready records: Real-time logs provide a transparent history of actions for regulatory inspections
- Security & integrity: Data is securely stored, ensuring protection against unauthorised modifications
With Lumi, organisations are always prepared for audits, reducing the stress and workload associated with compliance reporting.
Step 4: Continuous monitoring & optimisation
Post-implementation, Lumi continues to provide value by:
- Generating actionable insights based on recorded data
- Highlighting deviations from standard procedures
- Offering analytics for process improvements
Regular system reviews ensure that Lumi remains aligned with evolving compliance requirements and operational goals.
Conclusion
By automating data capture and ensuring full traceability, Lumi transforms compliance from a reactive process into an integrated operational standard. Organisations that implement Lumi benefit from enhanced data integrity, reduced manual workload, and a streamlined approach to regulatory adherence. With Lumi, compliance is not just maintained – it becomes a fundamental strength of laboratory and manufacturing operations.